A federal science agency has released a new report as part of its effort to ensure that cannabis products are accurately tested and labeled for THC, CBD and more than a dozen other cannabinoids. It’s part of an ongoing effort by the National Institute of Standards and Technology (NIST) to encourage standardized analysis of a wide range of cannabis compounds and contaminants across an ever-expanding class of legal products.
The agency, part of the U.S. Department of Commerce, announced The Cannabis Quality Assurance Program (CannaQAP) last summer. The program’s goal, NIST said at the time, was “to help laboratories accurately measure key chemical compounds in marijuana, hemp and other cannabis products including oils, edibles, tinctures and balms.”
Laboratory testing of cannabis has been happening more or less openly in the U.S. for years, at least since the early days of medical marijuana dispensaries in California. While NIST has acknowledged that most existing product labels already included concentrations of at least THC and CBD, the agency says many labs don’t have sufficient experience conducting those tests, which has led to “unreliable” results.
NIST already guides standardized testing and measurement in other industries, such as dietary supplements and food safety. “We work already in this space with regulators, product manufacturers, farmers, on the forensic side as well as in other areas,” NIST research chemist Brent Wilson told Marijuana Moment in a recent interview. “We had the interaction with them already, and we knew that they needed us to get involved to help improve the analytical testing [of cannabis] that’s being done in the community.”
The first exercise in the CannaQAP program, which is the subject of the new report released last week, involved evaluating how laboratories determine the concentration of cannabinoids in hemp oils. Testing laboratories across the country were sent two samples of hemp oils containing known concentrations of THC, CBD and 15 other cannabinoids. After testing the samples, the labs returned their results to NIST along with an explanation of their testing methods.
The primary goal of the first report, published July 27, is meant to show how much variability exists between testing labs and methods. Its goal is to be observational and educational, not to pass judgment on the labs techniques or measurements. It published results in anonymized form, looking for how much measurements varied. “There needs to be a platform for these laboratories to demonstrate their confidence without worrying about…failing or passing a test that they’re taking part in,” Wilson said.
Overall, 116 laboratories participated, although not all reported results for each sample, nor did all submit results for each cannabinoid contained in the samples they analyzed. According to a list of self-identified participants, the group comprised an international mix of commercial labs that already focus on cannabis testing, commercial or academic chemical testing labs, law enforcement agencies and assorted others. Overall, about 83 percent returned data to NIST, Wilson said.
“The industry as a whole compared pretty well with the target values,” he said of the overall THC and CBD results covered in the report. “The accuracy as a community was good.”
Because the quality assurance program is intended to be anonymous and nonjudgmental, Wilson declined to draw any significant critical conclusions about the significance of the variability between laboratories’ results. Asked how the group’s cannabis results compared to those of other regulated industries NIST works with, he said the report showed “very similar results to what we’d expect in any of the similar programs that we’ve done. There’s always going to be a large amount of variability at first [but] as things progress, we expect to see that variability shrink.”
“We generally don’t go into the details about the capabilities of an industry,” he added, explaining that job is better left to the U.S. Department of Agriculture or state regulators. “Our main goal is just to try to improve the measurement capabilities.”
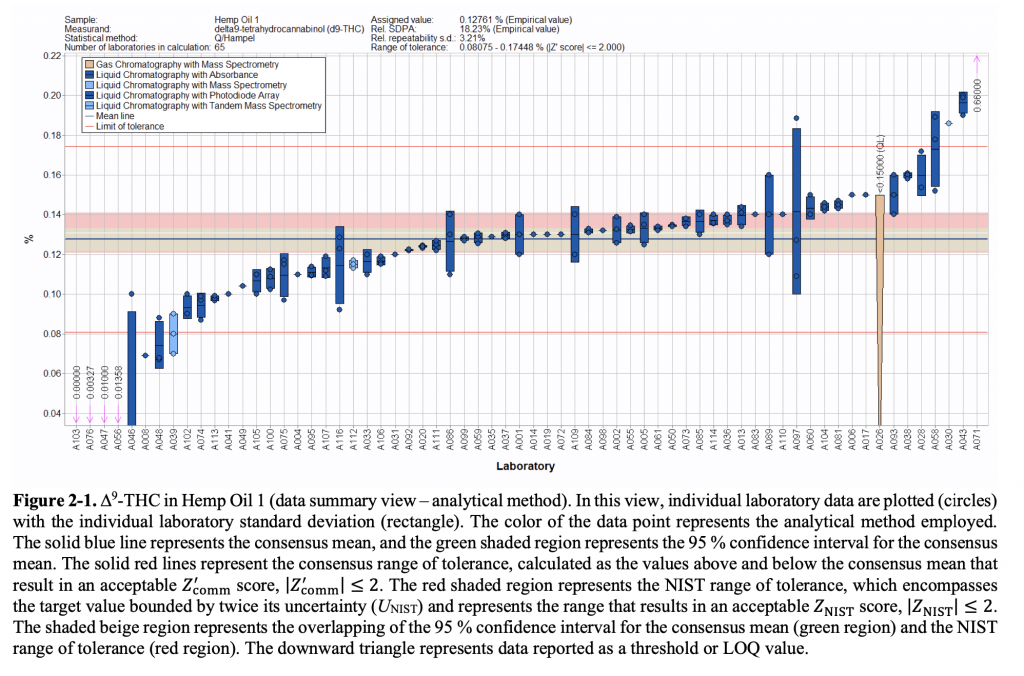
Results of laboratories’ testing for delta-9 THC in a hemp oil sample (via NIST)
Wilson did draw a few broad conclusions about the quality assurance results. For one, laboratories overall generally clustered around the intended measurement of the test samples when testing for THC. But some returned results that were significantly above or below the target value. By comparison, labs that returned test results for CBD also clustered around the accurate result, but those that missed the mark tended to fall below the intended value. Compared to THC, Wilson added, CBD results also showed “a fairly wide range of variability.”
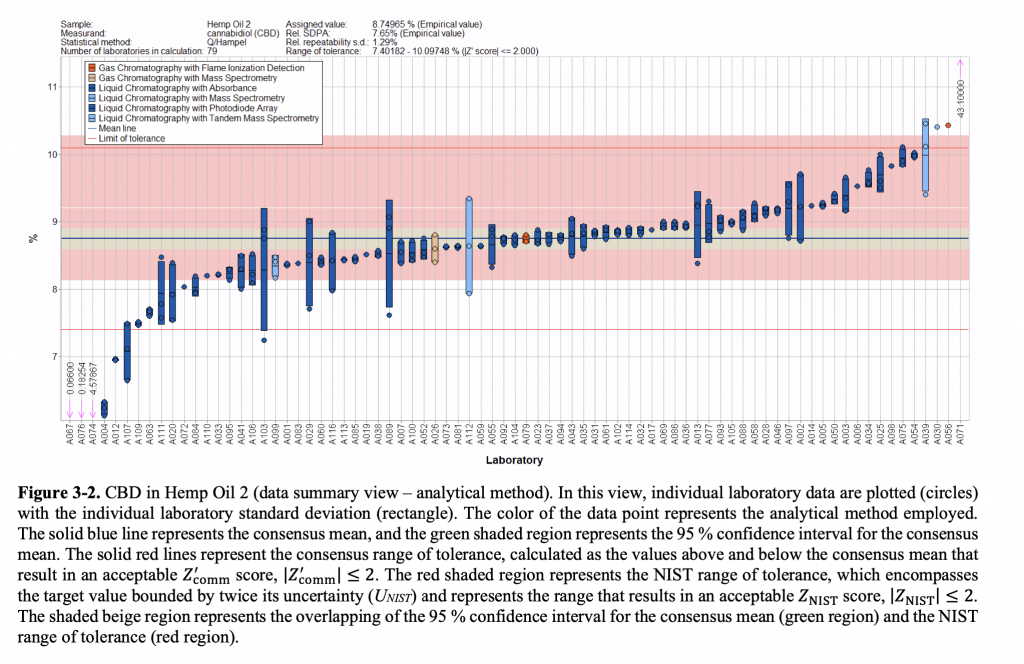
Results of laboratories’ testing for CBD in a hemp oil sample (via NIST)
Wilson attributed the difference between the two patterns as likely resulting from differences in calibration when conducting testing, though he said other differences—such as testing method or storage conditions prior to testing—may also explain the results. “The variability is there across all analytes being measured,” he said—which is to be expected.
“Everybody wants a true value for something, but the truth is that the true value is unknown,” Wilson emphasized. “Somebody tells you the concentration is 0.3 percent—that’s the estimated value. They don’t know what the true value is.”
Every testing method—and every test itself—has minor differences that affect the end result such that it’s impossible to eliminate any margin of error. Test a sample four times, and even the best tests will return minutely different numbers each time, he said. “There’s always some type of uncertainty associated with that.”
One obstacle NIST faces in interpreting some of the results, Wilson said, is that in some cases a vast majority of labs, up to 93 percent, said they used similar testing methods for some compounds.
“They used very similar types of methods,” he observed, making it harder to identify how differences in testing method might influence results. “It makes it more difficult to understand if it’s the method.”
NIST plans to conduct a similar test again in about a year to see how results compare to the first round of quality assurance testing. “Let’s test you and see how you do on the exact same matrix,” Wilson explained. “Have you improved your measurement capabilities from a year ago? … Part of the goal is just getting labs thinking about this and seeing what they can improve moving forward.”
The second of CannaQAP’s exercises, which is still being prepared for publication, will focus on cannabis plant material and examine not only cannabinoids but also testing for moisture and toxic contaminants such as heavy metals. Exercise three has yet to be announced, but Wilson said he expected more information to be announced later this month. “We do have some things that we’re planning to include, but I can’t really divulge that right now.”
As part of its overall cannabis testing program, NIST is also working to develop a hemp reference material with known concentrations of various compounds, which the agency says labs could use to validate their testing methods. Currently no such cannabis reference material exists.
A key motivation for the program as a whole was the 2018 Farm Bill, which legalized hemp across the country. Crucially, it defined hemp as cannabis that contains less than 0.3 percent THC—an arbitrary distinction that sent law enforcement agencies and product manufacturers alike scrambling to test samples for compliance. “Labs can have a hard time distinguishing between the two because accurately measuring THC can be difficult, especially at such low levels,” NIST said in a January release.
“If you’re going to confiscate a farmer’s crop, or subject a person to prosecution,” Wilson said in a statement at the time, “you want to be sure that measurement is accurate.”
Exercise two of the quality-assurance program focuses on plant matter rather than homogenized oil, and it includes marijuana samples that exceed the 0.3 percent limit on THC in hemp. Participating laboratories—220 signed up—received six total samples, three of which were at or below the federal 0.3 percent cutoff, and three samples Wilson said were “around 0.3 to 2 percent” THC.
Testing a Schedule I controlled substance—any cannabis samples over 0.3 percent THC—”presents additional challenges on our part,” the researcher noted. For example, rather than simply shipping hemp samples, NIST has to ensure participating labs have the proper U.S. Drug Enforcement Administration paperwork so NIST can perform a legal transfer of marijuana.
As the program evolves, Wilson said NIST expects to use samples that have higher concentrations of THC “so we can target both hemp and marijuana companies that are out there to try to improve their capabilities.” He also said future studies will focus on the wide array of products that cannabinoids are added to, including edibles, topicals and a range of nutritional supplements.
“The entire cannabis space is still new in terms of testing,” Wilson said. One thing he’s heartened by is the broad consensus he says he’s seen among researchers, law enforcement and commercial testing labs on the need for improved accuracy and standardization in testing. “Across all aspects of the community, all of them want to improve,” he said. “The only path forward is together.”
New Marijuana And CBD Research Amendment Could Be Added To Senate’s Infrastructure Bill This Week
Medical Disclaimer:
The information provided in these blog posts is intended for general informational and educational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. The use of any information provided in these blog posts is solely at your own risk. The authors and the website do not recommend or endorse any specific products, treatments, or procedures mentioned. Reliance on any information in these blog posts is solely at your own discretion.